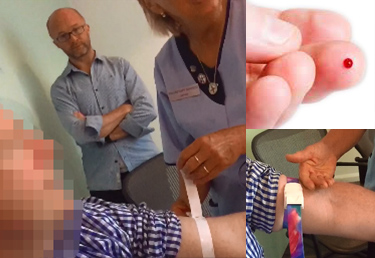
Medical Devices
Biomedical diagnostic testing coupled with connected medicine is set to change the face of healthcare management. Increasing sensitivity and sophistication of assay platforms is enabling a step change in diagnostic monitoring, transferring it from the traditional professional lab setting into doctors' surgeries and homes around the globe. Applications range from the management of common chronic diseases, such as diabetes, to monitoring health and wellbeing or preventative screening. This presents a number of design challenges to our clients.
Design Challenges
Connected medical device development is an area of expertise and focus at Marble. In an increasingly connected world, devices are no longer stand alone products. All product touch points need to be considered to ensure seamless integration with the entire service offering. Design must consider the entire system including; usability engineering, developing unique appeal, simplification of product and use concepts, miniaturisation of mechanisms, user interface design, software and electronics development, data management and transfer, and risk assessment. All working within the regulatory framework for the medical device.

Usability Engineering
An effective usability engineering approach requires a thorough preliminary analysis to ensure all users, environments, tasks and scenarios are considered in concept generation. Frequent and considered formative testing is undertaken throughout to ensure devices are safe, effective and easy to use, with risks and usage errors mitigated. Input and feedback during front end insight gathering and sketch concepts as well and the testing of physical prototypes and GUI is invaluable to ensure user requirements and regulatory obligations are met.
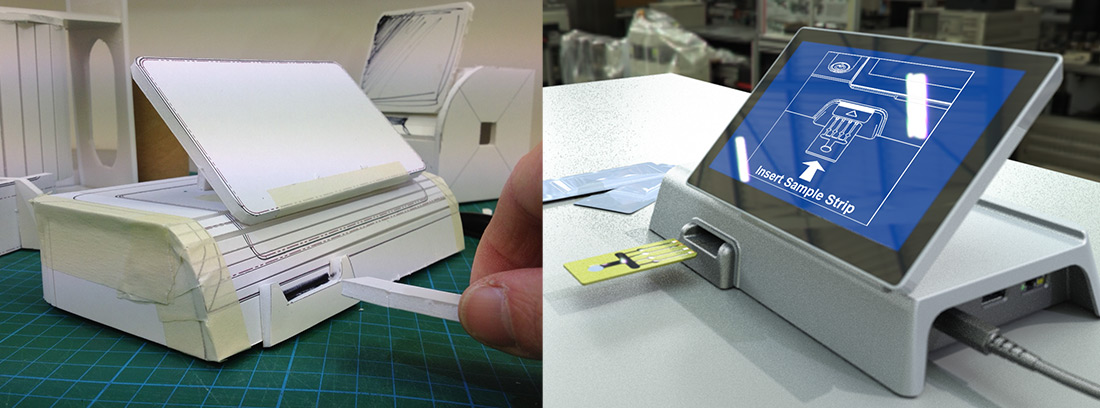
Prototyping and Visualisation
Having the right stimulus material at the right time is essential to ensure informed feedback and effective decision making. Thus enabling key product stakeholders, such as board members or investors, to fully understand the product proposal. Marble facilitates the development process by generating; sketches, block models, photorealistic renderings, high quality visuals and working prototypes. We utilise our in-house workshop and machinery, supplemented with a proven network of specialist suppliers.
Electronics and Software Integration
Developing the control elements for a medical product can be a costly and time critical element. At Marble we consider the controls architecture very early in the development process. We utilise off the shelf electronics and in-house software to prove critical aspects such as novel electro-mechanical devices, sensors and user interfaces in the early design phases. We build fully functional working prototypes based on standard processor boards with custom ancillary PCB's in order to more rapidly prove the product architecture. This is a fast and risk managed approach appropriate for early stage development prior to transition to fully custom electronics. Marble can select and work with a high volume manufacturer of custom medical device electronics to support the latter stages of device development.

Detailed Engineering and Design
Around 80% of the product cost is determined during the first 20% of the product development timeline. Successful product development means answering key questions correctly as early as possible. We undertake a full range of product development activities including DFM, DFA, FEA, mathematical modelling, mechanical engineering, CAD design, mouldflow, statistical tolerance analysis and testing. This ensures the design and assembly are optimised to meet design specifications, whilst eliminating inefficiency and waste for longer term commercial viability. We apply a tailored approach to every client and project to ensure it meets their specific needs.